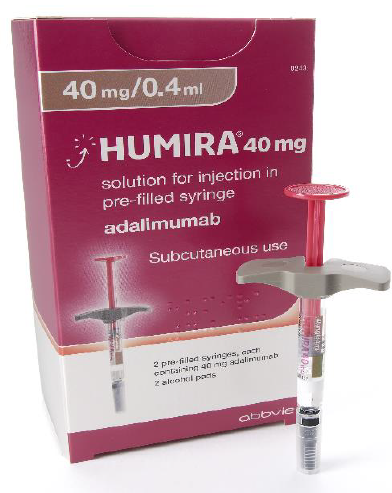
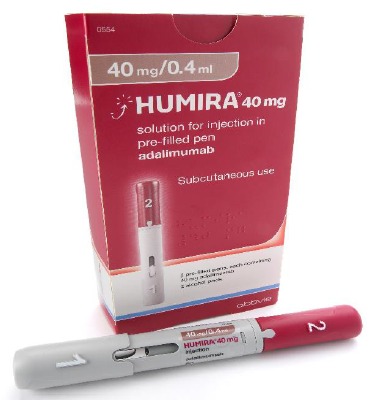
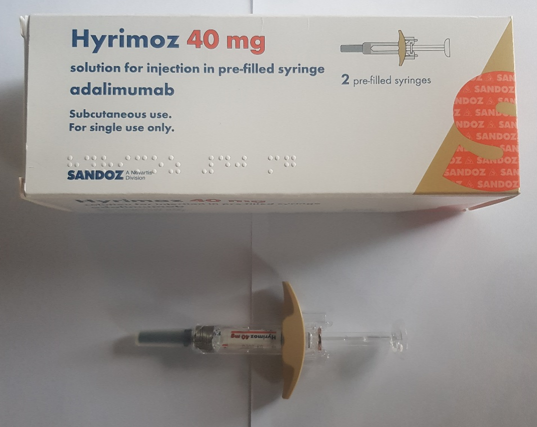
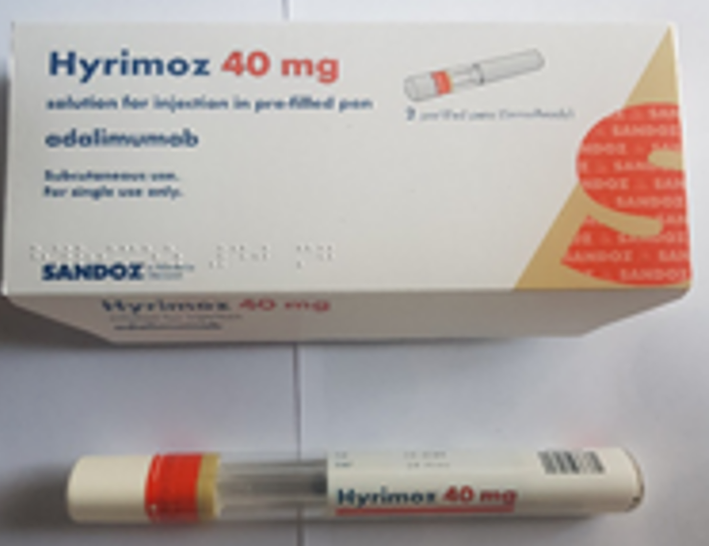
Considerations when choosing products
Several adalimumab products are now available for procurement by NHS Trusts.
Take the following factors in to account when deciding which adalimumab products to procure or prescribe.
For organisations
Ensure a local risk assessment is undertaken on the range of devices and strengths that are kept in the department to mitigate against mis-selection of products.
For individuals
Consider the devices or products that individuals can use in terms of manual dexterity and any potential allergies (e.g. latex).
Consideration should also be given to other pre-filled pen devices that individuals may be currently using to ensure they can differentiate between the intended adalimumab device to be used.
Products available
The adalimumab biosimilar contracted products are available in pre-filled syringes or pre-filled pens.
Pre-filled syringes
The following characteristics are common to all the available adalimumab biosimilar pre-filled syringe products:
- the package and syringe are clearly labelled with the brand-name prominent, and clearly differentiated between other brands of adalimumab
- have a PIL with a clear diagrammatic guide to administration
- use a standard plunger action to administer the dose
Humira® is the only product with a needle that does not retract after administration.
Pre-filled pens
The following characteristics are common to all the available adalimumab biosimilar pre-filled syringe pens:
- the package and pens are clearly labelled
- have a PIL with a clear diagrammatic guide to administration
- after injection, the needle retracts within a sleeve
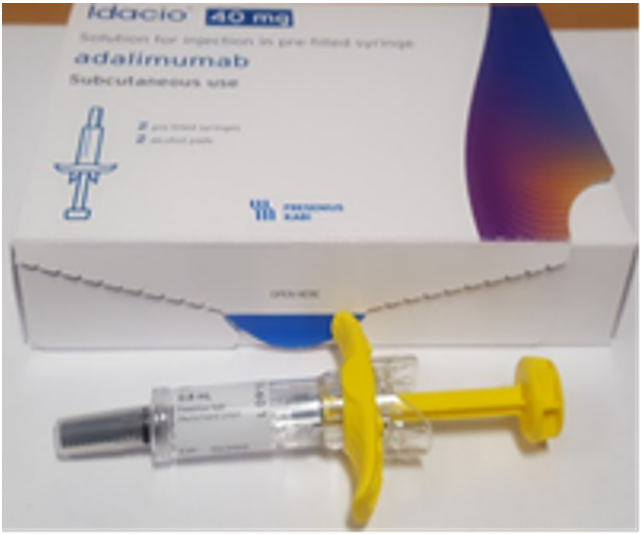
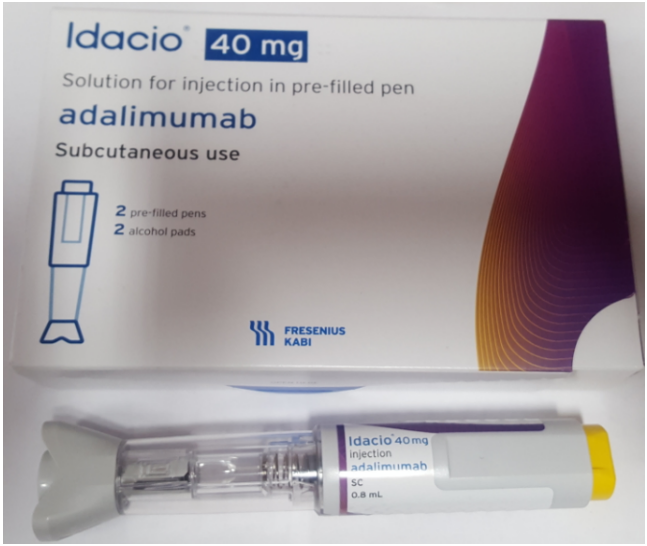
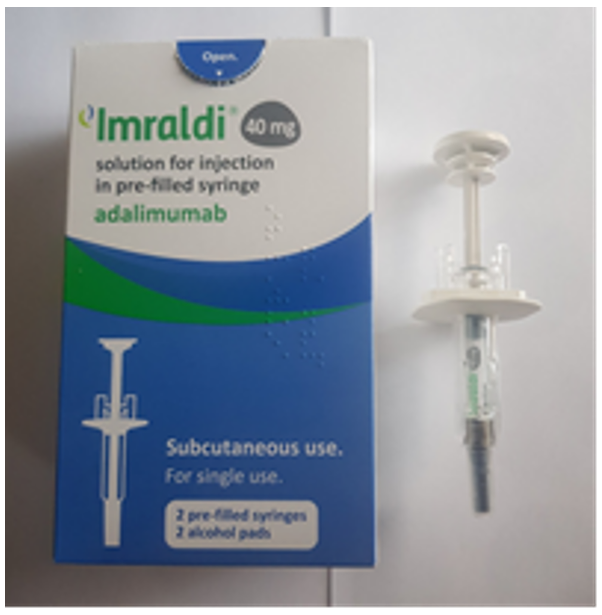
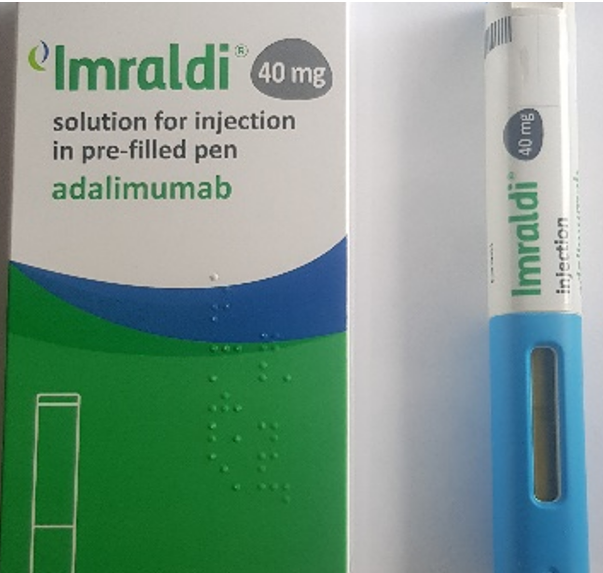
Product images and PILs
Images have been provided to help mitigate against product mis-selection.
Patient Information Leaflets (PILs) are available from the electronic Medicines Compendium or MHRA Products and can support discussions between healthcare professionals and individuals.
Safety and efficacy
Safety and efficacy of biological and biosimilar medicines is covered in our article on understanding biological and biosimilar medicines.
Licensed indications
All adalimumab biosimilar products (regardless of strength) are licensed for the same indications as Humira 40mg with one exception: Hyrimoz is not licensed for paediatric ulcerative colitis.
Humira 20mg is licensed for paediatric indications and Humira 80mg is licensed for a selection of adult and paediatric indications.
Consult product information for further details.
Injection site pain
Pain associated with subcutaneous administration can be caused by several factors and the factors may be additive.
The differences in needle gauge or injection volume across the different adalimumab biosimilar products are not significantly different in their potential to cause injection site pain.
Injection volume
Injection volume for subcutaneous administration should ideally be below 1.5mL and volumes below 0.8mL is associated with reduced pain. As long as the volume remains below 0.8mL, further reduction in volume may not significantly reduce pain.
The following adalimumab products are formulation as 40mg in 0.8mL:
- Amgevita
- Hyrimoz
- Idacio
- Imraldi
The following adalimumab products are formulation as 40mg in 0.4mL:
- Humira
- Yuflyma
Needle characteristics
Needle diameter can affect injection site pain: smaller diameter needles cause less pain. However, all of the needles used with adalimumab preparations are small gauge, 27G (0.413mm) or 29G (0.337mm), and the small differences are unlikely to cause a difference in practice.
The following adalimumab products have 29G needles:
- Amgevita – pre-filled syringes
- Humira – pre-filled pens and pre-filled syringes
- Idacio – pre-filled pens and pre-filled syringes
- Imraldi – pre-filled pens and pre-filled syringes
- Yuflyma – pre-filled pens
- Hyrimoz – pre-filled pens and pre-filled syringes
The following adalimumab products have 27G needles:
- Amgevita – pre-filled pens
Buffer
Buffers are added to parenteral formulations to adjust and control the pH. The strength of buffer, the pH and the nature of the buffer all affect injection site pain. However, appropriate balance of buffers in the formulation should ensure products are not unnecessarily painful at the point of injection.
Citrate
Citrate buffers, especially at high concentrations, are particularly associated with injection site pain compared to acetate, phosphate and histidine.
Individuals who have experienced injection site pain with adalimumab may benefit from trying a citrate-free product.
The following products are citrate-free:
- Amgevita
- Humira
- Yufluma
The following products contain citrate:
- Hyrimoz
- Idacio
- Imraldi
Allergens
Latex
The following products are not manufactured with latex as a raw material. However, the manufacturers cannot guarantee that minute amounts of latex are not contained in raw materials obtained from their suppliers and cannot guarantee that the product has not come into contact with latex during the manufacturing process:
- Amgevita
- Humira
- Hyrimoz
- Imraldi
- Idacio
- Yuflyma
Mannitol
The following products contain mannitol:
- Amgevita
- Humira
- Hyrimoz
- Idacio
Polysorbate
All the available adalimumab products contain polysorbate 80, except Imraldi, which contains polysorbate 20.
Other excipients of interest
Sorbitol
Imraldi is the only product that contain sorbitol.
Sucrose
Amgevita is the only product that contains sucrose.
In-use stability
All adalimumab products should be stored in the refrigerator. However, products differ in the time they may be kept out of the fridge when in use.
Up to 14 days
The following products can be stored at 25°C for up to 14 days:
- Humira – pre-filled pens and pre-filled syringes
- Amgevita – pre-filled pens and pre-filled syringes
Up to 21 days
The following products can be stored at 25°C for up to 21 days:
- Hyrimoz – pre-filled pens and pre-filled syringes
Up to 28 days
The following products can be stored at 25°C for up to 28 days:
- Imraldi – pre-filled pens and pre-filled syringes
- Idacio – pre-filled pens and pre-filled syringes
Up to 30 days
The following products can be stored at 25°C for up to 30 days:
- Yuflyma – pre-filled pens
Comparison summary
The following summary is an abbreviated version of this article and has been provided to support procurement discussions.
Update history
- Amgevita is no longer manufactured with natural rubber (latex). Text and attachment have been updated.
- Updated Hyrimoz entry to change needle gauge from 27G to 29G and deleted reference to Hyrimoz 20mg pre-filled syringe which is not marketed in the UK
- Updated to reflect Hyrimoz products are not formulated with a latex-containing substance and can be considered latex-free. Comparison summary table replaced with V1.1
- Published